We unleash your business potential by maximize the business innovation.
Send EmailCalcium Hypochlorite, Calcium Oxychloride, Calcium Dihypochlorite, Calcium Chlorohypochlorite, Calcium Chlorohydrochlorite, 7778-54-3
CAS: 7778-54-3
Molecular Formula: Ca.2ClHO
Names and Identifiers
| Name | Calcium hypochlorite |
| Synonyms | Bleaching powder hypochlorous acid Calcium hypochlorite calcium dihypochlorite Calciumhypochloritetech calciumb11306hypochlorite calciumchlorohypochloride calciumchlorohydrochlorite Calcium Hypochlorite, Purified Calcium hypochlorite technical grade |
| CAS | 7778-54-3 |
| EINECS | 231-908-7 |
| InChI | InChI=1/Ca.ClO/c;1-2/q+2;-1 |
| InChIKey | ZKQDCIXGCQPQNV-UHFFFAOYSA-N |
Physico-chemical Properties
| Molecular Formula | Ca.2ClHO |
| Molar Mass | 142.98 |
| Density | 2.35g/mLat 25°C(lit.) |
| Melting Point | 100°C(lit.) |
| Water Solubility | 200 g/L (20 ºC) (dec.) |
| Solubility | 200g/l (decomposition) |
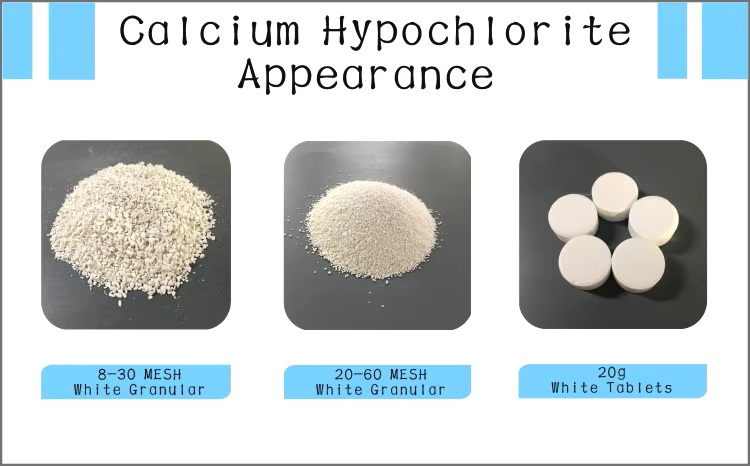
| Appearance | Tablets |
| Specific Gravity | 2.35 |
| Color | White to off-white or faint green |
| Odor | Chlorine-like odor |
| Merck | 14,1674 |
| Storage Condition | Store at +15°C to +25°C. |
| Stability | Stability Strong oxidizer - contact with flammable material may lead to fire. Incompatible with water, reducing agents, combustible material, phenol. |
| Physical and Chemical Properties | Character white powder. Has a chlorine-like odor. solubility dissolved in water. |
| Use | Used for cotton, hemp, Pulp, silk fabric bleaching, drinking water, swimming pool water sterilization and Disinfection, purification of acetylene |
Risk and Safety
| Risk Codes | R8 - Contact with combustible material may cause fire R22 - Harmful if swallowed R31 - Contact with acids liberates toxic gas R34 - Causes burns R50 - Very Toxic to aquatic organisms |
| Safety Description | S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S61 - Avoid release to the environment. Refer to special instructions / safety data sheets. |
| UN IDs | UN 1748 5.1/PG 2 |
| WGK Germany | 2 |
| RTECS | NH3485000 |
| TSCA | Yes |
| HS Code | 2828 10 00 |
| Hazard Class | 5.1 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 850 mg/kg LD50 dermal Rabbit > 2000 mg/kg |
Upstream Downstream Industry
| Raw Materials | Chlorine Calcium hydroxide Calcium oxide |
Nature
A white powder with a chlorine-like odor. Dissolved in water, its aqueous solution is alkaline. Exposed to air, easy to absorb moisture, carbon dioxide and decomposition of the release of hypochlorous acid and chlorine, hypochlorous acid with the decomposition of hydrogen chloride and gaseous oxygen. Easy to absorb moisture, the nature is extremely unstable; Sunlight, heat, acidity make it deteriorate and reduce the effective chlorine content. Mixed with organic matter, flammable liquid can Fever spontaneous combustion, by high heat will be an explosion, toxic.
Preparation Method
- calcium method: first, the limestone and the white coal are pressed by 1 :( 0. The ratio of 11 to 0.13) is intermittently added from the top of the lime kiln to the lime kiln for calcination, and the calcination temperature is 800 to 1200 ° C. The generated lime is intermittently discharged from the bottom of the kiln, and water is added to it for digestion, the slaked lime containing a small amount of free water is obtained, which is aged for more than 8 days, and is sent to the air separation system for slag removal through the auger. And the powdered slaked lime is separated by the cyclone separator. The fine-grained slaked lime containing 3-6% of free water is fed from the fourth layer of the chlorination Tower via a dragon under the slaked lime bin, and chlorine gas (liquid chlorine liquefied tail gas) is fed from the first layer of the chlorination tower, the finished bleaching powder is discharged from the first layer of the chlorination tower. The tail gas discharged from the chlorination tower is absorbed with liquid alkali to generate sodium hypochlorite, and the waste gas is vented.
- sodium method: after the selected calcium oxide is digested with water and part of the mother liquor (from the subsequent process), the digestion emulsion is continuously filtered and used for standby; The filter residue is treated with hydrochloric acid to form calcium chloride, acidification pH value is 6~9. Chlorination is carried out in two consecutive cycles: the digestion emulsion enters the first reaction tank, and chlorine gas and caustic soda solution are introduced into the tank, and the first chlorination reaction is carried out in order to complete the chlorination reaction, the overflowing primary chlorination liquid enters the second reaction tank for secondary chlorination. A large amount of heat was evolved during the reaction, and chilled brine was immediately removed to control the reaction temperature. The reaction solution flowing from the secondary chlorination tank was passed through a Salter, and sodium chloride was separated and flowed out from the bottom of the Salter by using the difference between the crystal particle sizes of calcium hypochlorite and sodium chloride. The slurry in the upper part of the saltformer is separated to obtain a filter cake of calcium hypochlorite, which is dehydrated and dried to obtain a finished product, and the mother liquor is returned to digest calcium oxide.
Use
- An oxidizing bactericide whose bactericidal mechanism is similar to that of liquid chlorine. Long-term use of the product will increase the system Caz concentration.
- is a highly effective bleaching agent. It is mainly used for bleaching of cotton and hemp textiles, chemical fibers, pulp and starch, biochemical treatment of pollutants, etc. It is also used for Disinfection and sterilization of drinking water and swimming pool water. Military can be used as chemical agents (such as mustard gas, etc.) and radioactive disinfectants.
Safety
toxic, irritating to the skin and eyes, entering can cause Nasal Congestion, sore throat. Contact and use of the staff should wear the provisions of equipment, to prevent ammonium hypochlorite solution into the human body and skin, if inadvertently into the body, should be immediately washed with water. When the skin is burned, it should be washed with dilute solution of sodium thiosulfate and water, and then coated with olive oil. Calcium hypochlorite with organic matter, flammable substances, can spontaneous combustion. It shall not be stored and mixed with organic matter, acids, oils and reducing agents.
Reference Information
| Introduction | bleaching powder chemical formula CaCl2 · Ca (ClO)2 · 2H2O, white powder substance, the composition thereof varies with the preparation conditions, and is generally a hydrated double salt composed of calcium hypochlorite, calcium chloride and unreacted calcium hydroxide, and the active ingredient thereof is calcium hypochlorite. Bleaching powder has strong oxidation, corrosion and irritation. When water, ethanol or inorganic acid can be decomposed, when water or inorganic acid, it is decomposed into hypochlorous acid, and hypochlorous acid has strong oxidation, bleaching and sterilization properties, therefore, the bleaching effect of bleaching powder is mainly based on the oxidation of hypochlorous acid. |
| sterilization mechanism | The bleaching powder dissolves in water and releases hypochlorous acid. HCIO acts on the protein of the pathogen and causes the protein to be denatured and lethal, easy to decompose into atomic oxygen, atomic oxygen has strong oxidation, can oxidize the pathogen protein and kill the pathogen; Hypochlorous acid can also explain the release of chlorine (CI ), directly acting on the pathogen protein, denaturation of protein to death; Plus calcium chloride solution in bleaching powder solution is strong alkaline, can dissolve Virus protein shell, is conducive to the release of Virus particles and death. Bleaching powder Disinfection object is very extensive, Virus, bacteria, fungi, micro particle spores have a killing effect. Can also be used for silkworm room, silkworm, silkworm, silkworm seat, silkworm eggs, mulberry leaves and other Disinfection. |
| Application | bleaching powder can be used for bleaching cotton, hemp, pulp, and is also a cheap and effective disinfectant and insecticide. |
| preparation | bleaching powder can be prepared by passing chlorine gas into slaked lime. The reaction formula is as follows: |
| toxicity | This product dust and the release of chlorine on the respiratory tract, skin and eyes have a strong stimulating effect. In case of accidental splash into the eye, it should be washed with water immediately. Touch the skin to wash with water, such as the skin is burned when the application of dilute sodium thiosulfate solution and water wash, and applied olive oil. The operators should wear work clothes, anti-virus masks, closed glasses, latex gloves and other labor protection articles. |
| Use | bleaching of cotton, hemp, pulp and silk fabrics, drinking water, sterilization of swimming pool water and Disinfection, purification of acetylene and so on mainly used for bleaching of cotton fabric, chemical fiber, Pulp, also used for Disinfection, sterilization and so on used as analytical reagents, bleaching agent, depilatory agent and oxidizing agent used for bleaching cotton, hemp, pulp and silk fabrics, sterilization of drinking water, swimming pool water and Disinfection, This product is a highly efficient bleaching agent for the purification of acetylene. Storage at room temperature for more than 200 days does not decompose. It is mainly used for bleaching of cotton, fiber, pulp and starch. Can also be used for drinking water, swimming pool water Disinfection and sterilization and leather storage in the mold preservative. is a highly effective bleaching agent. Mainly used for bleaching of cotton and hemp textiles, chemical fibers, Pulp, starch, also used for drinking water, swimming pool water Disinfection and sterilization. Military can be used as chemical agents (such as mustard gas, etc.) and radioactive disinfectants. It is mainly used for bleaching pulp in paper industry and bleaching cotton, hemp and silk fabrics in textile industry. Also used for urban and rural drinking water, swimming pool water sterilization Disinfection. The chemical industry is used for the purification of acetylene, the manufacture of chloroform and other organic chemical raw materials. It can be used as wool anti-shrinkage agent, deodorant, etc. antimicrobial agent; Tap water purifying agent; Disinfectant; Bleach. It is the same as bleaching powder, but the content of available chlorine is high. When dissolved in water, less insoluble residue, stable nature, there is a tendency to replace bleaching powder. disinfectant, bleach, deodorant, oxidant. |
| production method | chlorine gas is obtained by passing into milk of lime, then centrifuged, dried and pulverized. By chlorine gas into the mixture of sodium hydroxide and calcium hydroxide obtained. Known as sodium method. absorption method the limestone and white coal are added to the lime kiln from the top of the Lime Kiln intermittently according to the ratio of 1 (0.11~0.13), and the temperature is controlled at 800~1200 ℃, the generated lime is intermittently discharged from the bottom of the kiln, and it is digested by adding water to obtain slaked lime containing a small amount of free water, which is aged for more than 8 days and sent to the air dressing system for slag removal through the auger. And the powdered slaked lime is separated by the cyclone separator. The fine-grained slaked lime containing 3-6% of free water is fed from the fourth layer of the chlorination Tower via a dragon under the slaked lime bin, and chlorine gas (liquid chlorine liquefied tail gas) is fed from the first layer of the chlorination tower, the finished bleaching powder is discharged from the first layer of the chlorination tower. The tail gas of 2Ca(OH)2 2Cl2 → Ca(Ocl)2 · CaCl2 · 2H2O discharged from the chlorination tower is absorbed with liquid alkali to generate sodium hypochlorite, and the waste gas is then vented. The lime produced by calcination of limestone by milk of lime chloride method is crushed, digested, air-separated and mixed into a chlorination reaction vessel, and then subjected to chlorination reaction with human chlorine gas to generate bleaching powder concentrate, followed by centrifugal separation, drying and grinding, the finished bleaching powder concentrate was prepared. Its 8Ca(OH)2 6C12 → this product 3CaCl2 4H2O put lime into a lime kiln to burn lime. After crushing, 1 430 kg is put into the digester, water is added and slurry is mixed into the chlorination reactor, and 990 of chlorine is encountered. After full reaction, the solid is dried and ground to obtain the product. |
| category | oxidant |
| toxicity grade | poisoning |
| Acute toxicity | oral-rat LD50: 850 mg/kg |
| explosive hazard characteristics | explosion under water heat |
| flammability hazard characteristics | Fever flammable in case of organic fuel; Decomposition of combustion-supporting oxygen in case of acid; toxic chloride smoke with high thermal decomposition |
| storage and transportation characteristics | The warehouse is ventilated and dry; Light loading and unloading, separate storage of sulfur and phosphorus combustibles |
| extinguishing agent | Water |