We unleash your business potential by maximize the business innovation.
Send EmailE951, Aspartame, Dipeptide Sweetener, Aspartic Acid Phenylalanine Methyl Ester, 22839-47-0
Physical and Chemical Properties:
-
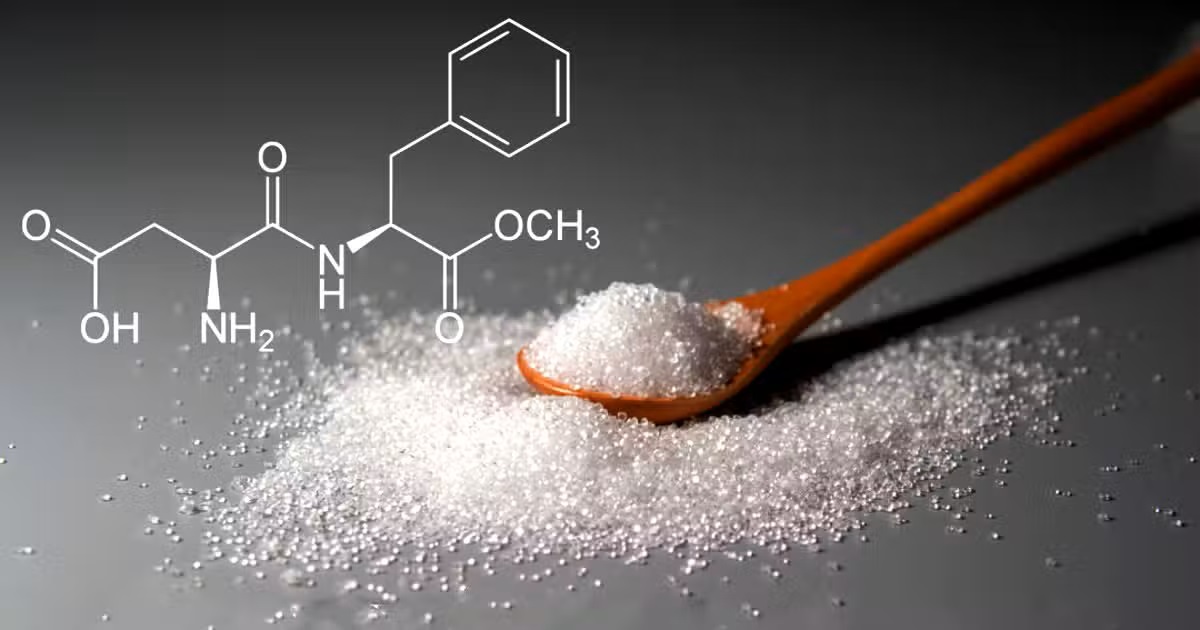
Molecular Formula: C₁₄H₁₈N₂O₅
-
Molecular Weight: Approximately 294.31 g/mol
-
Appearance: Typically a white, crystalline powder
-
Solubility: Easily soluble in water but poorly soluble in alcohol
-
Melting Point: Decomposes at around 246–247 °C (does not melt completely)
-
Chemical Nature: Aspartame is a dipeptide methyl ester, composed of aspartic acid and phenylalanine amino acids.
-
pH Sensitivity: Stable in acidic and neutral pH, but degrades in high temperature and alkaline conditions.
Other Names for Aspartame:
Aspartame is known by various names depending on scientific, technical, or commercial contexts:
-
E951 (European food additive code)
-
Aspartic Acid Phenylalanine Methyl Ester
-
L-Aspartyl-L-Phenylalanine Methyl Ester
-
Dipeptide Sweetener
-
Asp-Phe Methyl Ester
-
Methyl Aspartylphenylalanate
Comparison with Other Artificial Sweeteners:
-
Sweetness Level:
-
Aspartame is about 200 times sweeter than sugar.
-
Sucralose is 600 times sweeter than sugar, making it more potent.
-
Saccharin is 300–400 times sweeter than sugar.
-
-
Calorie Content:
-
Aspartame is low-calorie but not completely calorie-free.
-
Sucralose and saccharin are generally considered calorie-free.
-
-
Heat Resistance:
-
Aspartame is not heat-stable, limiting its use in baking and cooking.
-
Sucralose is heat-stable and suitable for high-temperature applications.
-
-
Health Considerations:
-
Aspartame contains phenylalanine, making it unsuitable for individuals with phenylketonuria (PKU).
-
Saccharin may leave a metallic aftertaste for some people, and its safety has been debated in the past.
-
Health Effects of Aspartame:
-
General Safety: Most health authorities state that consuming aspartame within the acceptable daily intake (40 mg per kg body weight) is safe.
-
Phenylketonuria (PKU): Individuals with PKU cannot metabolize phenylalanine and must avoid aspartame.
-
Neurological Effects: Some people report headaches or mood changes after consuming aspartame, but these effects vary by individual.
-
Cancer Risk: The WHO classifies aspartame as a "possible carcinogen," but evidence is limited, particularly for humans.
-
Gut Health: Aspartame might negatively affect gut microbiota, though more research is needed.
Recent Research on Aspartame:
-
Cancer Studies: The WHO and the International Agency for Research on Cancer (IARC) have identified aspartame as a "potential carcinogen," but only in cases of extremely high consumption.
-
Weight Management: According to the WHO, artificial sweeteners like aspartame may not effectively support long-term weight control or obesity prevention.
-
Uses and Safety: While aspartame is widely used in sugar-free beverages and low-calorie products, excessive consumption could pose health risks.
Molecular Formula (Aspartame): C14H18N2O5
Molecular Weight: 294.30 g/mol
Chemical Name: Aspartame
CAS Number: 22839-47-0
This chemical substance is a chemical compound in the artificial sweetener group developed by a chemist in 1965 while doing research for its use in ulcer treatment.
It is a substance whose chemical formula is the methyl ester of Aspartic Acid and phenylalanine.
E Code = E951.
Other Names:
Asp-Phe Methyl Ester
Methyl Aspartylphenylalanate
Dipeptide Sweetener
Aspartic Acid Phenylalanine Methyl Ester
L-Aspartyl-L-Phenylalanine Methyl Ester
Physical and Chemical Properties:
It is in crystalline powder form in terms of physical appearance. It has a taste that is approximately 200 times sweeter than the sucrose compound.
It has no odor.
In the presence of moisture, it is hydrolyzed by separating into aspartylphenylalanine compound and another chemical compound. This hydrolysis event causes a decrease in the taste of aspartame.
Melting Point is 346.5 °C.
Solubility varies according to temperature, pH and the presence of water. It has the highest solubility in low pH environments and at 25 °C. For example, when the pH is around 2 and the temperature is 25 °C, it was determined as 20 mg/mlt water.
It has a stable feature in the absence of stability. However, it loses its taste in the presence of moisture.
Aspartame Usage Areas:
It is used as an artificial sweetener in carbonated drinks in some countries.
It is used as a sweetener in the production of chewing gum thanks to its high sweetness feature.
It is used to provide sweetness in tea, coffee and some breakfast requirements.
It is used in the production of low-calorie foods and beverages. In this application, it is used either alone or mixed with another sweetener.
It is used as a sweetener in the production of ice cream.
It is used as a sweetener in cough syrups in the field of pharmacology.
It is used in chewable tablets and sugar-free liquid formulations.
It is used in the production of gelatin to give a sweet taste.